Proper lubrication of a mechanical system can make or break a design. Any application in which rubbing occurs requires lubrication to prevent excessive wear, heat buildup, seizing, and—in worst case scenarios—complete system failure. Lubrication concerns range from
- lubricant type
- lubricant quantity
- lubrication intervals
- lubricant degradation
- water contamination
…the list goes on.
Self-lubricating materials have not only eliminated these concerns for many designers of severe mechanical systems, but have opened up a new realm of applications which were previously unattainable. Eliminating the need for an external lubrication source allows engineers to design rubbing components in applications where the presence of lubrication would be inconvenient or impossible. Carbon graphite in particular has enabled engineers to advance a wide array of new applications.
Carbon Graphite Materials on the Molecular Level
Carbon graphite is arguably the most versatile of all self-lubricating materials, as it can be exposed to incredibly harsh conditions like extreme temperatures and caustic chemicals but still perform reliably. We can attribute all of this to carbon graphite’s molecular structure.
The carbon element can exist in three forms—diamond, graphite, and amorphous carbon. On a molecular level, these three forms differ only in the amount of valence electrons that are bonded. Of carbon’s four valence electrons, diamond has all four bonded, graphite has three bonded, and amorphous carbon has only two bonded.
On a macroscopic level, this results in drastically differing material properties, which is evident by looking at the materials side-by-side.
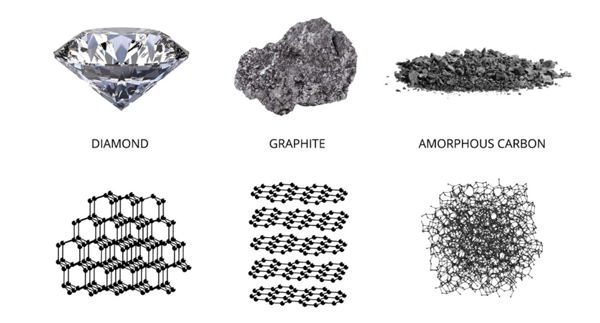
- Diamond: Four bonded valence electrons. Highly ordered lattice structure
- Graphite: Three bonded valence electron. Strong graphene layers bound together by weak van der Waals forces
- Amorphous Carbon: Two bonded valence electrons. Strong “entanglement” of carbons
Diamond is an incredibly hard material and, due to its scarcity in nature, is quite valuable. However, mechanical carbon graphite manufacturers are primarily concerned with the other two carbon forms: graphite and amorphous carbon.
Carbon graphite consists of an amorphous carbon matrix with graphite grains embedded within that matrix. Although the amorphous carbon provides most of the material’s strength, the graphite is what which makes the material self-lubricating.
Graphite: The Incredible Self-Lubricating Material
Graphite has a layered structure consisting of very strong graphene layers. These layers are bound to each other by weak intermolecular forces called van der Waals forces. Because van der Waals forces are so weak, the strong layers can slide quite easily on top of each other. This sliding action is the mechanism behind carbon graphite’s ability to self-lubricate.
Carbon graphite is a relatively soft material; on the Moh’s scratch hardness scale, carbon graphite is only a 2, right above talcum powder. This plays to carbon graphite’s advantage in terms of self-lubricity. When rubbing against a hard counter face, carbon graphite will deposit a burnished transfer film on the surface it is running against, essentially allowing the carbon graphite to take on the finish of that surface.
This means that the surface of the carbon graphite component is basically running against a burnished film of carbon graphite, not against the counter face itself. This helps tremendously in reducing wear rates, since there will be very little kinetic friction between two materials with the same surface finish. In order to maximize this effect, it is important that the counter face is as smooth and hard as possible.
In submerged applications, self-lubrication is bolstered even further through the formation of a hydrodynamic film. This hydrodynamic film is similar to the burnished transfer film in that it acts as a barrier between the carbon graphite and its counter face. It is such a good barrier, in fact, that the coefficient of friction in submerged applications is about a tenth of that in dry running applications (which is a pretty low number to begin with*).
Jack of All Applications
The benefits of self-lubrication are evident in the fact that carbon graphite is the material of choice in a wide variety of applications. The fact that such drastically different industries all benefit from this material is a testament to its versatility.
| INDUSTRY | CARBON GRAPHITE APPLICATION | BENEFITS OF SELF-LUBRICATION |
| Gypsum/Veneer Board Manufacturers | Board Dryer Bearings | Constant lubrication and maintenance of the bearings that support hundreds of rollers throughout the dryers can be a burden. Carbon graphite bearings offer an easy, maintenance-free alternative. |
| Jet Engine Manufacturers | Shaft Seals | No need for oil and grease lubricants, which would simply burn off at the extreme operating temperatures of these turbines. |
| Food/Pharmaceutical Conveyor Manufacturers | Conveyor Bearings | Avoid exposure of food/pharmaceutical products to grease or oil lubricants. |
| Air Compressor Manufacturers | Piston Rings | The compression system is free from suspended grease or oils. |
New carbon graphite applications are constantly in development. As a result, it is important that carbon graphite grades are developed at the same rate. There will always be a need for stronger, more chemically resistant, and higher temperature rated materials. Carbon graphite manufacturers are aware of this need and are focusing on this development. These manufacturers may have the right material for your specific application—all you need to do is ask.
*µDRY ≈ 0.05-0.30, µWET ≈ 0.01-0.05